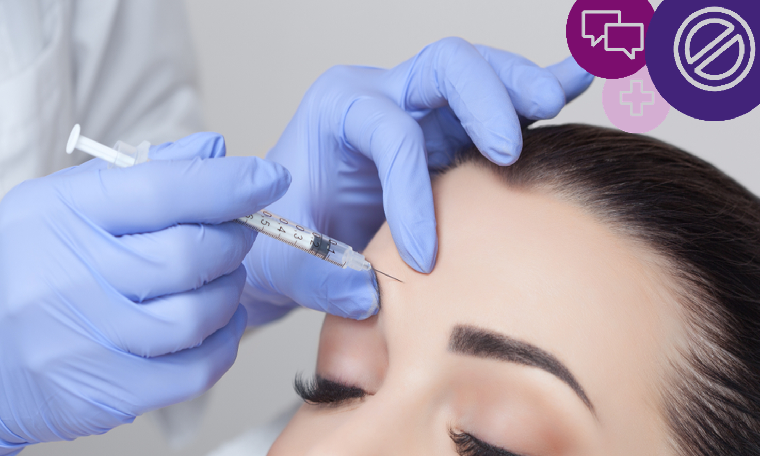
More and more of us are considering the cosmetic effects of Botox or similar products, all of which temporarily relax facial muscles to smooth out lines and wrinkles.
Practitioners understandably want to promote their services enticingly to potential customers. But although commonly marketed for their cosmetic effect, Botox and similar products (brand names include Vistabel, Dysport, Bocouture and Azzalure), are prescription-only medicines, used to treat medical conditions. Neither the products, nor treatments using them, can be advertised to the general public (Rule 12.2).
The prohibition includes logos, testimonials, links, hover text, small print and price lists and applies in all media covered by the CAP Code. The ASA regularly upholds against ads promoting Botox either directly or indirectly, including recently when Instagram posts featuring Kim Kardashian and Kylie Jenner both broke this rule (and others).
Approaches found to breach the Code include describing the benefits of Botox in the context of a Botox party; claiming to treat areas other than those for which the product is licensed and using endorsements by health professionals.
So what CAN you say?
It IS permissible to advertise directly to healthcare professionals in media addressed only to them. They are defined as people “qualified to prescribe or supply” medicines as defined by the Human Medicines Regulations 2012. The permission does not extend to beauty practitioners, unless they also have those qualifications, and ads must not appear in media targeted more broadly and likely to be seen by the general public.
If you are a clinic or pharmacy and Botox is one of several facial treatments you offer, you CAN promote consultations to the general public using wording such as “a consultation for the treatment of lines and wrinkles.” However, it must be clear that discussion of various treatment options will take place and a product won’t be sold or administered if a customer isn’t deemed suitable.
In the context of a website, very limited references to Botox may be acceptable as long as they do not appear on the home page and claims do not go beyond balanced and factual information typically found in the patient information leaflet, ‘Summary of Product Characteristics’ or similar non-promotional information that comes with the product. Casually browsing consumers must not be able to come across information relating to Botox or other POMs with ease.
So, with care, it is possible to market your services to the public. This AdviceOnline entry summarises the main points you need to consider and the CAP Copy Advice team will be happy to advise you on any specific queries – more details here.
More on
-
Keep up to date
Sign up to our rulings, newsletters and emargoed access for Press. Subscribe now.


